
Canada Appeals for International Firefighting Aid
June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces
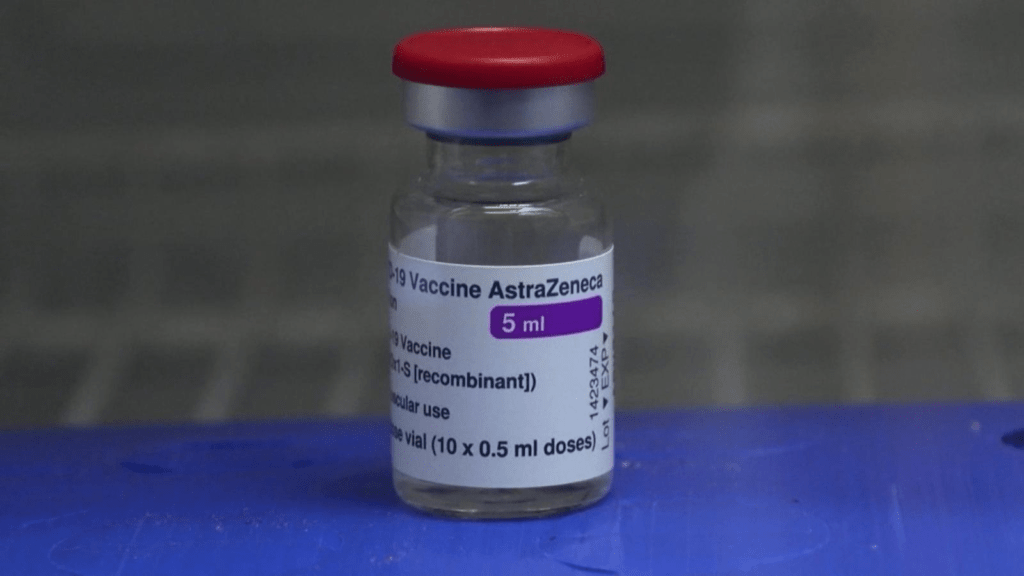
May 10, 2021: -People under the 40-year-old in the U.K. will be offered an alternative to the Oxford-AstraZeneca Covid-19 vaccine, as concerns regarding rare blood clots keep weighing on its rollout.
On Friday, speaking at a press conference, Professor Wei Shen Lim, chair of the U.K.’s Joint Committee on Vaccination and Immunisation (JCVI), said the authority had made changes in its advice for adults below 40.
“Building on our previous advice, we now advise that adults from the age of 30 to 39, who are not vaccinated and do not have an underlying health condition that puts them at higher risk of severe Covid-19, should be preferentially offered an alternative to the AstraZeneca vaccine where this is possible,” he said, adding that this would only be the case where “no substantial delay to vaccination might arise.”
He also said that the AstraZeneca vaccine was more accessible to transport and store than some of the alternative vaccines approved for use, which would influence how the new advice for below 40s was applicable.
“In certain settings, it may be the only vaccine practical to offer and, in those circumstances, it should indeed be the preferred vaccine,” he added. He also said that the advice was also conditional on reasonable Covid-19 infection control, good availability of alternative vaccines, and a vital vaccination rolls out in the United Kingdom.
A minimal number of people with the AstraZeneca vaccine have gone through blood clots. The condition, health authorities describe as “extremely rare,” is characterized by blood clots with low platelet levels.
Up until April 28, the U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA) had almost 242 reports of major blood clots accompanied by low platelet counts due to the dose of the AstraZeneca vaccine.
About 10.5 cases of blood clots with low platelet counts per million first doses of the AstraZeneca vaccine. The medicines regulator said in the report on Covid-19 vaccine side effects. On Friday, Dr. June Raine, chief executive of the MHRA, said that the ratio fell to one in a million for second doses. The fatality rate was 20% of the rare blood clots.
We provide the insights on leaders who are responsible for taking their organization to new heights, all the while bringing together a group of talented individuals.

June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces

May 27, 2025: Air Canada Cuts Five U.S. Routes for Winter 2025–26, Part of Broader Cross-Border Retrenchment

May 26, 2025: Trump Freezes $2.2B in Federal Grants to Harvard Over DEI, Threatens Tax-Exempt Status.

May 14, 2025: Microsoft has announced plans to reduce its global workforce by approximately 3%, affecting roughly 10,000 employees across multiple departments.

May 13, 2025: The Trump administration is considering suspending the constitutional right of habeas corpus in a bid to accelerate mass deportations.

April 29, 2025: Donald Trump’s second term has reached the 100-day mark under sustained public skepticism, with national approval ratings