
Canada Appeals for International Firefighting Aid
June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces
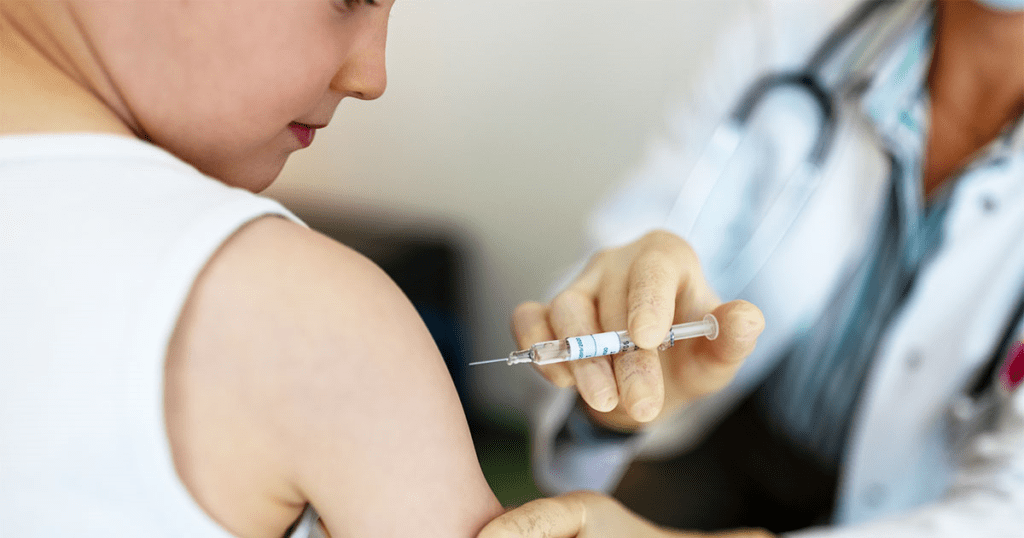
April 01 2021:-On Wednesday, Pfizer said its Covid-19 vaccine was robustly effective in kids, demonstrating 100% efficacy in phase three clinical trial testing its shot in adolescents aged 12 to 15.
Pfizer CEO Albert Bourla said the company is planning to submit the new data, which is developed with BioNTech, to the Food and Drug Administration and other regulators, hoping that kids in the age group will get vaccinated before the start of the new school year.
“We share the urgency to expand the authorization of our vaccine to use in young people, and the clinical trial data encourages adolescents between the ages of 12 and 15,” Bourla said in a press release.
The trial enrolled 2,260 participants in the U.S. There were 18 confirmed coronavirus cases observed in the placebo group and no confirmed cases in the group that received the vaccine, the company said. That resulted in a 100% vaccine efficacy, it said, adding that the shot was also well-tolerated, with side effects consistent with those seen in adults.
Children make up around 20% of the U.S. population, according to government data. Between 70% and 85% of the U.S. population needs to be vaccinated against Covid to achieve herd immunity, experts say, and some adults may refuse to get the shots.
Pfizer’s vaccine has been authorized already for use in the U.S. in people 16 and older. Clinical trial studies testing the vaccine in kids whose immune systems can respond differently compared to adults still needed to be completed.
The White House’s chief medical advisor, Dr. Anthony Fauci, told a House committee that the U.S. could start vaccinating older kids against Covid-19 this fall. At the same time, elementary-aged children may start getting their shots early next year.
Moderna, which also has a vaccine authorized in the U.S., said on March 16 that it had begun testing its shot in children under age 12.
Pfizer announced last week it started a clinical trial testing its Covid-19 vaccine on healthy 6-month to 11-year-old children.
For the first phase of that trial, the company will identify the preferred dosing level for three age groups, between 6 months and two years old, 2 and 5, and from 5 through age 11. According to the company, the kids will begin by receiving a 10-microgram dose of the vaccine before progressively moving to higher doses. Participants also have the option to take three microgram doses.
We provide the insights on leaders who are responsible for taking their organization to new heights, all the while bringing together a group of talented individuals.

June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces

May 27, 2025: Air Canada Cuts Five U.S. Routes for Winter 2025–26, Part of Broader Cross-Border Retrenchment

May 26, 2025: Trump Freezes $2.2B in Federal Grants to Harvard Over DEI, Threatens Tax-Exempt Status.

May 14, 2025: Microsoft has announced plans to reduce its global workforce by approximately 3%, affecting roughly 10,000 employees across multiple departments.

May 13, 2025: The Trump administration is considering suspending the constitutional right of habeas corpus in a bid to accelerate mass deportations.

April 29, 2025: Donald Trump’s second term has reached the 100-day mark under sustained public skepticism, with national approval ratings