
Canada Appeals for International Firefighting Aid
June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces
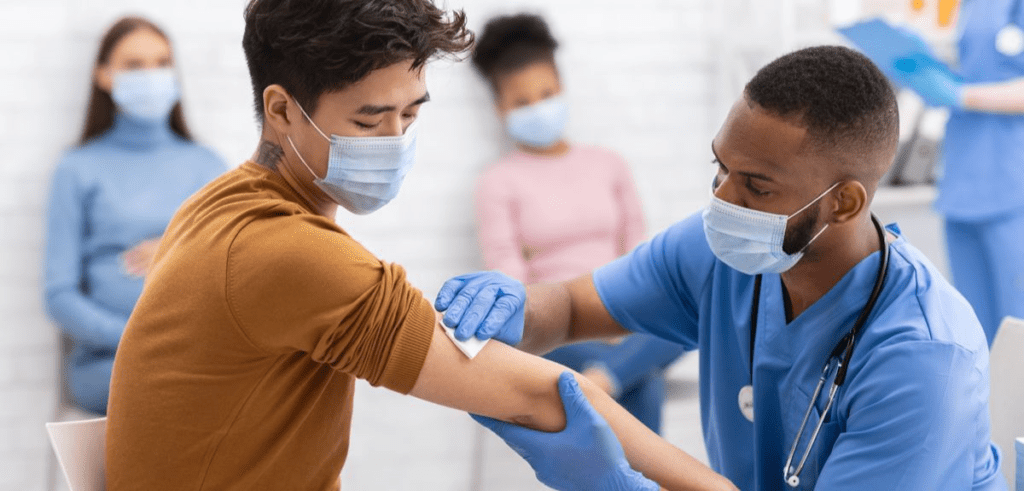
June 11, 2021: -On Thursday, Moderna said that it had asked the Food and Drug Administration to expand the emergency use of its Covid-19 vaccine in adolescents ages 12 to 17.
If approved by the FDA, it would likely dramatically expand the number of doses available to middle and high school students ahead of the coming school year. Pfizer and German partner BioNTech were cleared last month to use their vaccine for 12-to-15-year-olds.
“We are pleased to announce that we have submitted for an emergency use authorization for our COVID-19 vaccine with the FDA for use in adolescents in the United States,” Moderna CEO Stephane Bancel said in a press release. “We are encouraged that the Moderna COVID-19 vaccine was very much effective at preventing COVID-19 and SARS-CoV-2 infection in adolescents.”
Moderna’s announcement comes the same day an FDA advisory panel is scheduled to hold a vaccine meeting in kids.
Moderna said on May 25 its Covid vaccine was 100% effective in a study of 12-to-17-year-olds, making it the second shot to demonstrate high efficacy in younger age groups. Moderna’s two-dose vaccine, which is given four weeks apart, is already authorized for adults.
The study the company cited included more than 3,700 adolescents. According to the company, no cases of Covid were observed in participants who received two doses of the vaccine, while four points were observed in the placebo group.
No huge safety concerns are identified, and side effects are generally consistent with those seen in an earlier trial of adults, the company said. Moderna said the most common side effects after the second dose were headache, fatigue, muscle pain, and chills.
U.S. regulators are expected to grant the request of Moderna for use in teens. The approval process could take about a month, just in time for fall classes. Pfizer and BioNTech requested expanded use of their doses in adolescents on April 9, for example, and were authorized by the FDA on May 10.
Moderna has already filed for authorization for adolescents in Canada and the EU.
Children make up around 20% of the total U.S. population, according to government data. As a result, a few 70% to 85% of the U.S. population needs to be vaccinated against Covid to get herd immunity, medical experts say, and some adults may refuse to call the shots. In addition, more experts say herd immunity is looking increasingly unlikely as coronavirus variants spread.
Vaccinating kids maybe hasten the return of in-person learning and after-school extracurricular activities such as sports, art, and music, health experts say.
The Centers for Disease Control and Prevention on May 28 eased its public health guidance for summer camps, saying fully vaccinated teens don’t need to wear masks or stay 6 feet away from others.
We provide the insights on leaders who are responsible for taking their organization to new heights, all the while bringing together a group of talented individuals.

June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces

May 27, 2025: Air Canada Cuts Five U.S. Routes for Winter 2025–26, Part of Broader Cross-Border Retrenchment

May 26, 2025: Trump Freezes $2.2B in Federal Grants to Harvard Over DEI, Threatens Tax-Exempt Status.

May 14, 2025: Microsoft has announced plans to reduce its global workforce by approximately 3%, affecting roughly 10,000 employees across multiple departments.

May 13, 2025: The Trump administration is considering suspending the constitutional right of habeas corpus in a bid to accelerate mass deportations.

April 29, 2025: Donald Trump’s second term has reached the 100-day mark under sustained public skepticism, with national approval ratings