
Canada Appeals for International Firefighting Aid
June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces
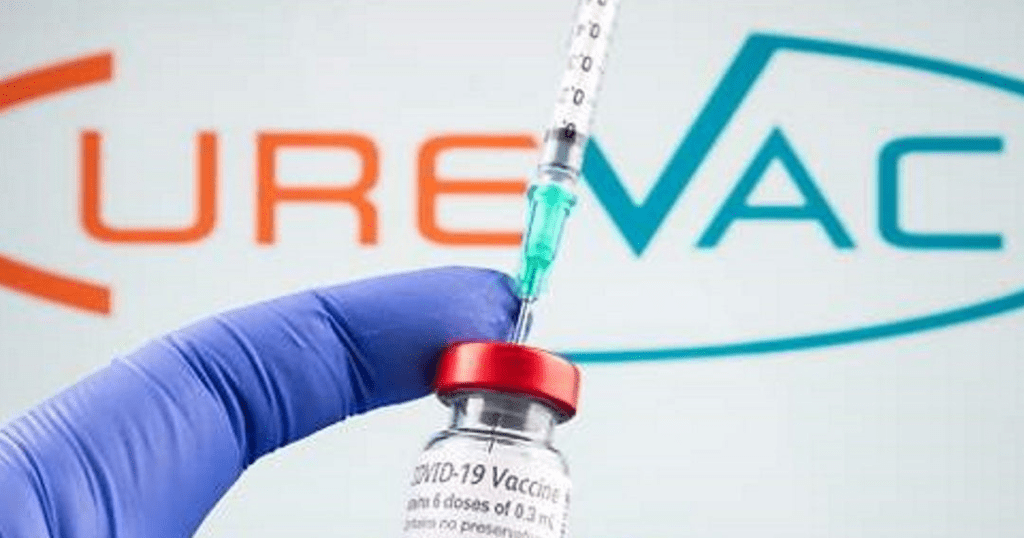
July 2, 2021: -CureVac plans to continue work on its Covid-19 vaccine despite disappointing clinical trial results that showed the shot is just 48% effective.
On Wednesday, the German biotech firm published its final analysis of the clinical trials of its coronavirus vaccine called CVnCoV, confirming that the shot was 48% effective against Covid of any severity across all age groups and 15 variants.
On Thursday, Pierre Kemula, CFO of CureVac, defended the vaccine on CNBC, however, saying the clinical trials had been conducted when multiple new strains of the virus were spreading across the world.
“We need now to speak to the European Medicines Agency (EMA) and want to make sure we have an open dialogue and share all the data we have to assess the path forward,” he told CNBC on Thursday.
When asked whether it was worth developing the vaccine when other successful shots were already deployed in Europe and elsewhere, Kemula said the company had to fulfill some contractual obligations.
“We have a contract with the European Commission to supply 225 million doses of the drug, so I think, with that in mind, we need to plow forward,” he said.
The results of the CureVac trial, involving 40,000 participants in ten countries in Latin America and Europe, proved the vaccine was more effective in younger people. The efficacy rate between those aged 18 to 60 came in at 53% against the disease of any severity and increased to 77% against moderate and severe infection in the same age of people.
However, as Covid-19 poses higher risks for older people, the trial results are disappointing, not least those from Pfizer-BioNTech and Moderna, which have proved to be above 90% effective at preventing coronavirus infection. Shares in CureVac dropped nearly 13% in pre-market trade on Thursday.
On Wednesday, CureVac CEO Dr. Franz-Werner Haas also defended the results in a statement, saying the vaccine “demonstrates a strong public health value” for those amid 18 and 60 and will be an “important contribution to help manage the Covid-19 pandemic and the dynamic variant spread.”
He cited “the current context of an increasingly diverse environment of Covid-19 variants.”
Multiple variants have emerged throughout the pandemic, with some more virulent than others. The alpha variant first discovered in the U.K., and the delta variant was first identified in India, and Kemula said he believed mutations would continue to occur.
“With more people infected with coronavirus, we are set for a continued evolution of the disease as it moves forward and has more variants,” Kemula added.
We provide the insights on leaders who are responsible for taking their organization to new heights, all the while bringing together a group of talented individuals.

June 09, 2025: Canada has issued an international appeal for firefighting support as wildfires intensify across multiple provinces

May 27, 2025: Air Canada Cuts Five U.S. Routes for Winter 2025–26, Part of Broader Cross-Border Retrenchment

May 26, 2025: Trump Freezes $2.2B in Federal Grants to Harvard Over DEI, Threatens Tax-Exempt Status.

May 14, 2025: Microsoft has announced plans to reduce its global workforce by approximately 3%, affecting roughly 10,000 employees across multiple departments.

May 13, 2025: The Trump administration is considering suspending the constitutional right of habeas corpus in a bid to accelerate mass deportations.

April 29, 2025: Donald Trump’s second term has reached the 100-day mark under sustained public skepticism, with national approval ratings